Growth and development of the brain
Let us look particularly at development of the neural circuits in the neocortex. This proceeds through a number of steps.
Neurogenesis
Formation of the brain’s neurons and glia takes place in the tissue of the telencephalon, specifically, in the vesicle walls. Neural stem cells in the ventricular (inner) zone proliferate by splitting via mitosis into two cells. At first, both daughter cells remain in the ventricular zone, populating the zone with neural stem cells. As the population goes up, the daughter cells more distant from the ventricular surface migrate upwards to populate the cortex. In this way, the neurons and glia of the cortex are formed from neural stem cells. In humans, most of this cell duplication occurs between the fifth week and the fifth month of pregnancy.
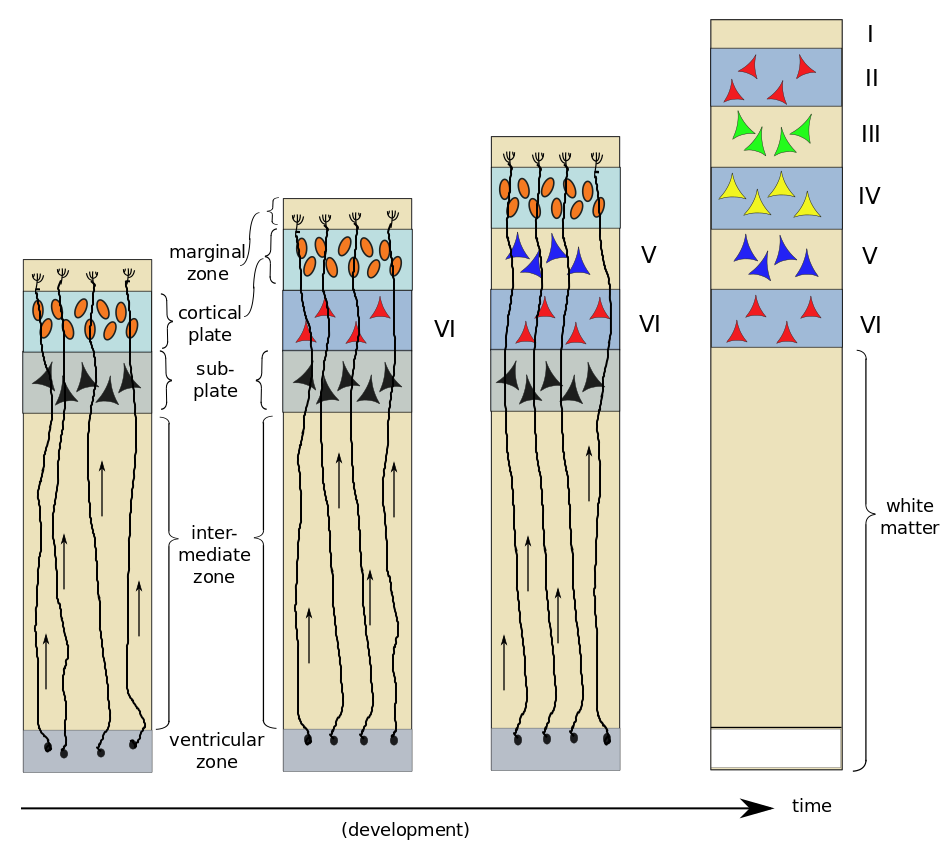
As an example, consider the six-layer striate cortex.[ref]Also known as V1, the primary visual cortex, in the occipital lobe.[/ref] First, radial glial cells extend processes toward the marginal zone; these will serve as “tracks” which migrating cells can follow. The young neurons, or neuroblasts, resemble ordinary cells, and do not yet possess neurites. About two thirds of them follow the radial glial cells upward towards the outer surface of the brain. The first to migrate form a subplate and begin to differentiate into neurons. The next neuroblasts pass through the subplate and arrive in the cortical plate where they start to differentiate in turn. In the case of the striate cortex, they will form layer VI[ref]That’s a Roman numeral “6”, not “Vee one”.[/ref], the innermost layer. The next neuroblasts pass by the Layer VI and start to form Layer V. This continues, with one layer being formed after another until all six cortical layers have been formed – in reverse order, or “inside-out”. After all six layers are formed, the subplate disappears and the radial glial cells withdraw the radial processes.
On arrival in their respective layers, the neuroblasts differentiate into pyramidal cells first. Afterwards, differentiation of glial cells takes place – first astrocytes and then oligodendrocytes. It has been found that neuroblasts already know what type of neuron they will become before arriving in their appropriate layer. A protein excreted in the marginal zone serves to attract pyramidal-cell dendrites but repel their axons, making them grow perpendicular to the surface and span several layers of cortex.
This laminar structuring of the cortex in horizontal layers formed by radial motion of neuroblasts explains the concept beloved of many neuroscientists of structures called cortical columns or minicolumns. This idea is based on the finding that discrete surface areas of cortex seem to communicate much more locally, using a leap-frog style of communication to contact more distant areas.[ref]Rather like the Internet’s Domain Name System.[/ref] Each such surface area is supposed to correspond to a column of cortical layers beneath it. Related to this is the concept that the cortex has pretty much the same cytoarchitecture everywhere, explaining its plasticity, wherein an area no longer needed for one function can be recruited for another. However, the utility of this concept has been much questioned in recent years, so its fate is not yet clear.[ref]See for instance “A brief biography of the cortical column”, or “The minicolumn hypothesis in neuroscience”.[/ref]
The other third of the neuroblasts, rather than migrating strictly outwards along the radial glial guides, move sideways also.
Most of the input from many parts of the body to the brain comes through the thalamus, which acts as dispatcher, so there are many thalamic inputs to the cortex. It seems that it is the subplate which attracts thalamic neurons to appropriate layers of cortex and thereby establishes which type of sensory input will be handled by that part of the cortex.[ref]See Bear, 697. This process and what it has to do with the non-radially migrating neurons is not clear to me.[/ref]
Synapse formation and wiring (“la connectique”)[ref]I couldn’t help using the french term here, as it is so simple.[/ref]
Growing neurites, which can be quite long and travel far in the body, advance as the growth cone at the tip of each one extends filopodia to drag it along an extracellular matrix of fibrous proteins. The axons tend to grow in groups of neurites stuck together[ref]“Axons which stick together grow together”?[/ref], each group dragged along step-by-step by one axon called the pioneer axon. The growth cone is attracted or repelled by various chemical substances already released during embyronic development. The concentration gradients of such chemicals guide the axons to their destinations. Further detail on connections is furnished by molecular surface markers on target cells; these markers are recognized by specific growing axons.
This method of connecting neurons is as remarkable as it is necessary. There are not enough coding genes in the human genome to specify all the connections of the 10 billion cells in our brains. What is specified are the functions – chemicals, markers and growth factors – which lead to such formation and connections.
In muscles, when the growth cone finally reaches its target, a neuro-muscular junction, a synapse is formed as follows. The growth cone secretes a protein which binds to a post-synaptic receptor. The receptor then does two things. It sets up the post-synaptic terminal by attracting ACh receptors (in the case of the NMJ) to the area. At the same time, it secretes Ca++ which acts on the pre-synaptic terminal in two ways, causing it to emit neurotransmitters and inducing modifications so that it takes on the form of a pre-synaptic terminal.
In the CNS, similar processes occur, but the steps are taken in a different order and different molecules are involved. Dendritic filopodia constantly reach out and around. When one comes into contact with a passing axon, pre-synaptic and post-synaptic terminals are installed and a synapse is formed.
Growing in this way, the nervous system comes to contain far too many neurons and, especially, connections. Neurons depend on limited amounts of substances called neurotrophic factors, which are furnished by target cells. It seems that cells are pre-programmed to commit apoptosis unless they are prevented from doing so by neurotrophins. Since there are not enough such resources available to maintain them all, pruning takes place, as some cells are left to commit suicide. Some neuroscientists see this as an evolutionary struggle for survival among neurons. Up through adolescence, axonal connections are rearranged, synapses used more develop and those used less disappear. This is one form of neuronal plasticity.
The strengthening of synapses will be considered in a later paragraph on learning and memory.
Go on to look at the parts of the brain.