The immune system
The immune system is complex, multi-leveled and multi-component.[ref]Its complexity and numerous components, which are not independent or separable for study, has led to confusion in the designation of its sub-systems. (Author’s opinion.)[/ref] Even though much has been learned about it in recent decades, it is still under intense investigation.
Rather than starting out with lists of the components, the structures and so forth, let’s look at an example.
Overview – physical barriers and inflammation
Suppose something bad – a virus or a mean bacteria – gets into a body. What happens then to protect against this pathogen?
The first line of defense against pathogens is largely mechanical or chemical. It comprises such things as the skin, which forms a natural barrier to keep things out; the mucus in our respiratory system, which captures microbes in the air we breathe and, by means of tiny hairs – cilia – drags them back up to where we can swallow them[ref]Yuk.[/ref]; and the gastrointestinal system, which is highly acid and can therefore kill many invaders.
But some of the invaders get past these barriers. And some do not even need to, because they show up internally. And tissue damage can result from external sources also, as when a hammer is injudiciously applied to a thumb. So after the barrier layer, what then?
The next step is often inflammation, called the inflammatory response. The infected area turns red, swells up, becomes hot and hurts. This region is the battlefield where the fight carries on after the barriers have been breached. This is where the various cells of the immune system go about protecting us.
Injured cells call for help by releasing chemical messengers called chemokines into the interstitial fluid. These indicate to cells farther away that there is a problem to be solved and implores their assistance. The cavalry arrives not by following a trumpet call, but by moving against the gradient of the chemokines, toward their point of greatest concentration, in a process called chemotaxis.[ref]Neutrophil to driver: “Follow that pathogen!”[/ref]
Among those coming to help, mast cells are one of a group of cells which contain granules, from which they release histamine, leukotrienes and prostaglandins. These chemicals cause vasodilation: Blood vessels expand so more blood can come in, bringing help, but they also become porous. Increased blood flow and plasma leakage into the interstitial space are responsible for the heat and redness and painful swelling.
Chemokines also lure in phagocytes to surround and absorb pathogens. The first ones to arrive are neutrophils, the most plentiful phagocyte in the body. Other phagocytes — macrophages and dendritic cells — come to phagocytize pathogens, to engulf them and destroy them in the process of phagocytosis.. All the cells mentioned so far are part of the non-specific or innate response. They are non-specific because each of them may act against any pathogen. Because they all rush in, they operate quickly, but they are neither very precise nor efficient.
Now we reach the next level of the immune system, the specific immune system, or adaptive immune response, as B cells and T cells begin to do their thing.
Any invading organism consists of at least one compound — in it or on it — which can be used to identify it uniquely. These compounds are called antigens, from antibody generations. The adaptive response depends on these markers to distinguish one pathogen from another. Phagocytes, like macrophages. dendritic cells or B cells, extract the antigens from the pathogen itself and display them on their own surface. This enables a specific response, one depending on the identity of the pathogen. Such a series of events takes more time than the innate response, but because it is specific to the particular pathogen, it is usually more effective.
Macrophages which display antigens on their surface serve as messengers or links between the innate and adaptive responses and begin the intricate process by which B cells, with the collaboration of T cells, produce antibodies, which can protect us over time.
The complement system is composed of proteins which aid immune response. They may, for instance, bind to a pathogen and thus label it – opsonization – calling attention to it so that phagocytes will deal with it. The complement system can help out both the non-specific and specific responses.
Now let’s look at these different immune-system components in more detail.
Innate immune response
The innate, or non-specific, immune system depends on several types of cells. It most likely evolved before the adaptive immune response.
One group of cells is constituted of phagocytes, which can engulf and contain pathogen cells, a process called phagocytosis. The contained pathogen, called a phagosome, is usually killed by a lysosome in the phagocyte cell. A phagocyte recognizes pathogens in a somewhat approximate way, as its surface (not to mention its genes) can contain only a certain number of antigen pattern recognition receptors (PRRs). Phagocytes are also important because they represent the first step in the process which identifies pathogens and leads to the release of specific antibodies. There are several phagocytes associated with the innate immune response.
- A macrophage is an amoeba-like phagocyte which can squeeze through tissues and capillary walls. It is capable of phagocytozing quite large bacteria.
- A neutrophil is a spherical phagocyte summoned from the blood stream. It is a granulocyte, meaning that it contains granules of substances such as histamine. It is the most abundant of the white blood cells (WBCs).
- A dendritic cell is an irregularly shaped cell with many appendages looking like the dendrites on neurons, hence the name. However, they are not neurons. Although they may be partly phagocytotic, their principal function is as antigen-presenting cells, about which more very soon.
- A monocyte is a precursor cell which may mature into either a macrophage or a dendritic cell.
A natural killer cell (NK) is not generally cosidered a phagocyte, but it is capable of convincing a pathogen cell to commit suicide, or apoptosis. It may do this through the use of a special ligand or by releasing perforin to bore a hole in the cell wall and then cause granzyme to enter the cell and bring about apoptosis.
Antigen-presenting cells (APCs)
The mechanisms of antigen presentation are somewhat complex. T cells, an essential part of the adaptive immune system, recognize antigens only when they are presented on the surface of antigen-presenting cells (APCs). APCs internalize the pathogen or antigen, break It up and bind pieces of it with a protein called the major histocompatibility complex (MHC) molecule. Only when the complex formed by the MHC molecule and the antigen is presented on the surface of the APC can it be recognized by T cells.
There are two types of MHCs and so two types of APCs.
- The so-called professional APCs are the innate-response macrophages and dendritic cells as well as the adaptive-response B cells, all of them cells of the immune system. When such a cell phagocytizes an external pathogen, it associates pieces of the antigens with MHC class II (MHC II) molecules and displays the complex on its surface.
- Non-professional APCs include all nucleated cells in the body.[ref]Reminder: red blood cells lose their nuclei.[/ref] They are not phagocytes, but they nevertheless display class I MHC (MHC I) complexes of internal pathogens, such as viruses which have reproduced within the cell.
Professional APCs can display MHCs of both classes, depending on whether the pathogen is internal (MHC I) or external (MHC II).
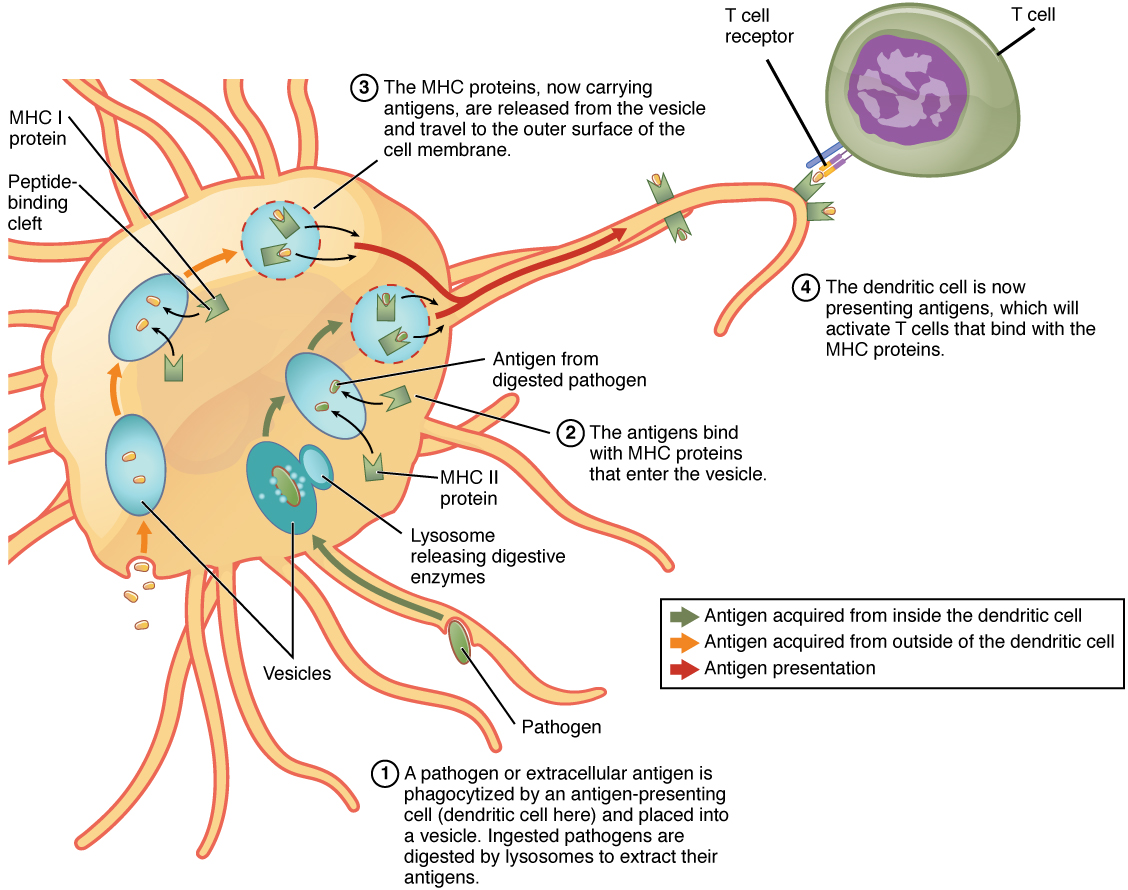
Antigen presentation, from Openstax College
Leukocytes, lymphocytes and WBCs
Blood cells are all differentiated from hematopoietic stem cells. For granulocytes like neutrophils, this occurs only in the marrow of bones . But lymphocytes and plasma cells are produced in lymphogenous material, which exists in lymph glands and other areas, including bone marrow
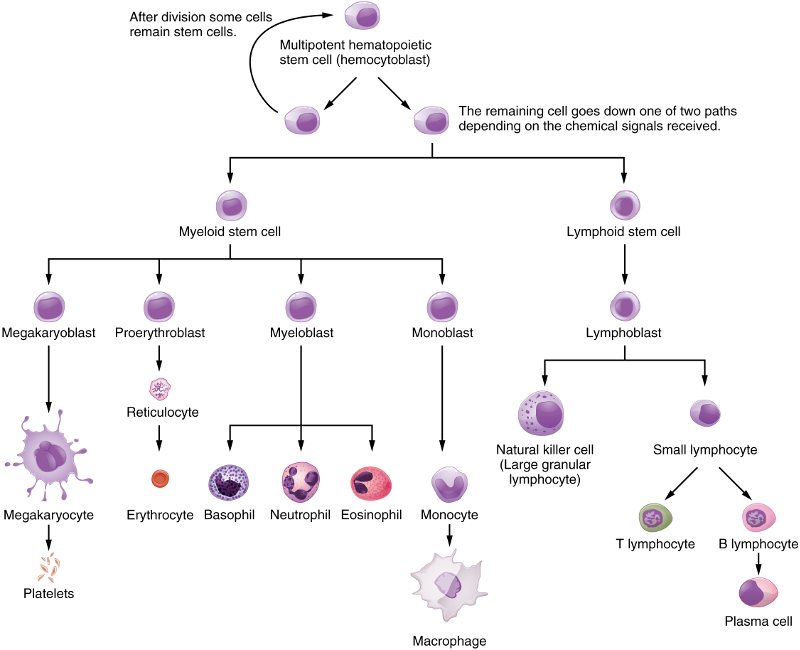
Differentiation of hematopoietic stem cells, from Openstax College
Erythrocytes are red-blood cells, of which the adults contain no nuclei, and platelets are responsible for blood clotting. The other cells shown are all WBCs, or leukocytes. In the lymphatic system, they are called lymph. They also constitute the interstitial fluid between cells.
Dendritic cells are special, existing in several varieties, and can come from a myeloid or a lymphoid precursor, not shown in the figure.
NK cells, B cells and T cells are called lymphocytes. Whereas the NK and B cells mature in bone marrow, T cells migrate to the thymus for maturation. NK cells are part of the innate immune system. B and T cells are cells of the adaptive immune system.
Each instance of a B or T cell has surface receptors which can be activated by only one type of antigen. Therefore, in order to detect almost any pathogen, B‑cell and T‑ell receptors exist in souch a great number of variiants that it would be impossible for so many genes to exist in their DNA. This difficulty is overcome by means of an astounding technique – gene shuffling. During the expression of their genes, segments of genes are shuffled like cards, giving over 1011 possible combinations, each of which detects a specific antigen.[ref]Isn’t this amazing?[/ref]
After strong selection against cells which may attack their own organisms, both types of cells migrate to lymphoid tissue throughout the body.
When a macrophage phagocytizes a pathogen, it passes antigens on to the lymphocytes. When a lymphocyte receives its specific antigen, it becomes activated and reproduces abundantly. (B cells also require the help of special T cells, as we shall see in a moment.) Activated B cells produce antibodies. Groups of antibodies or T cells activated by and sensitive to a specific pathogen are called clones.[ref]A clone is not a simple copy, it is a set of copies sensitive to a specific antigen.[/ref] Cells of a clone therefore share the same antigen receptors. Only lymphocytes which are activated by an antigen reproduce and multiply into clones, so the process can be seen as one of natural selection. Only activated cells survive and reproduce.
Adaptive immunity
Now for more details. I find this subject easier to understand by proceeding from less complex to more, so I will start with B cells, which will leave them partly unexplained until T cells have been discussed.
Humoral response – B cells and antibodies
When a “naive” B cell leaves the bone marrow or the lymphatic system and binds to an antigen, its ctivatiion process has begun. Part of the antigen is internalized, broken up and displayed on the cell’s surface in an MHC II complex. When this is bound by a type of T cell called a Th2 cell (the part that is left until later, but not much), the B cell is completely activated. (Some B cells are T-cell independent and do not require such activation.) The B cell then is duplicated into sets of clones to make many B cells of two types – effector cells and memory cells. The effector cells, now called plasma cells (not to be confused with blood plasma, which is mostly water) release a form of their surface receptors called antibodies, or immunoglobins (abbreviation Ig), into the environment.
The immunity conferred by antibodies is called humoral immunity. Each antibody recognizes only the antigen specific to its parent B cell. When antibodies – immunoglobins – encounter a pathogen carrying this antigen, they bind tightly to it via a lock-and-key mechanism based on their respective shapes and thus prevent the pathogen from doing any harm. They also signal phagocytes to absorb and kill the pathogen (opsonization again).
The memory cells wait around until they die or are needed again to clone and produce more receptor and memory cells. Some of them remain available for years or even decades. When needed, they allow for a much more rapid response to subsequent infections, called secondary response, as opposed to the initial, primary response. In secondary response, B cells as plasma cells are already available for producing antibodies without going through all the rigmarole of APCs and being activated by a T cell. We will look at why this takes place later.
Antibodies exist in five classes, imaginatively called, IgA, IgD, IgE, IgG and IgM.
- Only IgM and IgD function as receptors on a B cell; IgD remain on the B cells.
- IgM can leave the B-cell surface. They are the largest of the Igs, having 10 binding sites, whereas IgA has four and the others only two. So IgM is an excellent binder, especially during the early part of a primary response. In the latter part of the primary response, IgM can undergo a process of class switching in which it changes to a class IgG, IgA or IgE.
- IgG is the most common (80% of antibodies in serum) and is the most important antibody in secondary response. It also is the only one which can cross the placenta to protect the fetus.
- IgA has two forms. The eight-chain structure moves into mucous membranes and so is the only antibody to leave the interior of the body. The four-chain form remains in the blood.
- IgE is very rare. It serves to make mast-cell degranulation specific. In doing so, it contributes to allergies.
Cell-mediated response – T cells
Like B cells, T cells exist in some 1011 possible versions, each receptive to a specific antigen. But T cells are more complex (which is why we left them until now), being of two types, one of which has sub-types.
T cells can not detect antigens directly; they can only detect those which are displayed on the surface of APCs. Just as there are two types of APCs, according to their MHC type, there are two types of T cells. Certain glycoproteins on T cells are called clusters of differentiation, or CDs. On leaving the thymus, most T cells have either CD4 or CD8 and so are called CD4+ or CD8+.
- CD4 is a co-receptor which enables so-called helper T cells, or Th cells, to recognize class II MHCs on APCs, including those on B cells.
- CD8 is specific to MHC I and to cytotoxic T cells, also called Tc cells.
- There is a third type, called Treg, for regulatory T cell, with CD4 and CD25.
Tregs are less well understood, but suppress immune response by other T cells in certain cases.
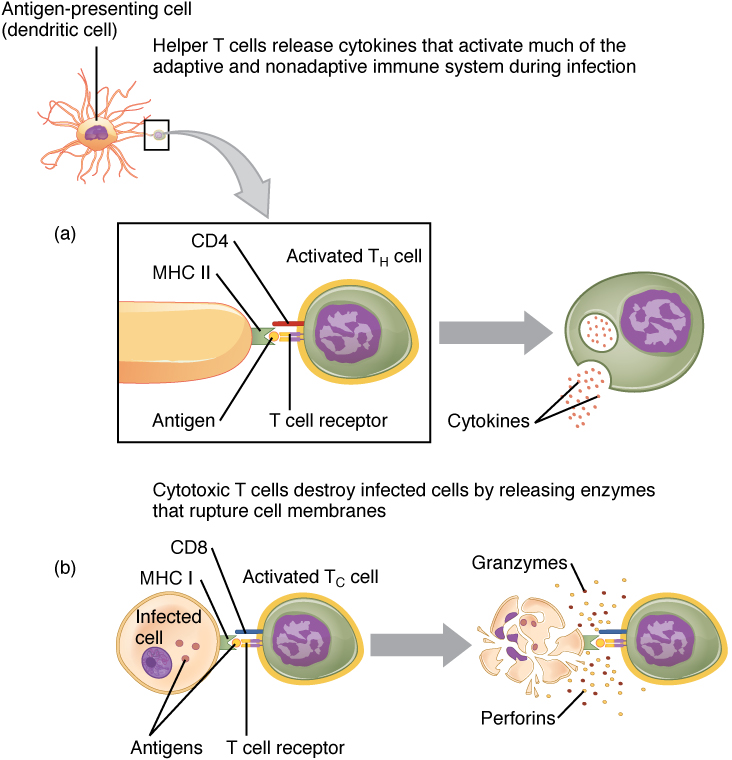
T-cell activation by APCs, from Openstax College
Of the two principal types, those simpler to understand are the CD8+ cells, called cytotoxic T cell or Tc cells when they are activated. As in the case of B cells, activated T cells multiply into sets called clones and while some of these become memory T cells, awaiting the next infection, the others become effector T cells. Effector T cells destroy infected cells by inducing apoptosis, like NK cells.
The other main type of T cell is the helper T cell, or Th, which exists in two versions, Th1 and Th2, the difference being mainly in the type of cytokines they secrete. Cytokines are short-distance signaling molecules. Those secreted by Th1 act as an alarm signal and promote phagocytosis. Alas, helper T cells are damaged by the human immunodeficiency virus (HIV).

B cell activation, from Openstax College
Th2 is the one we have been putting off and which allows the B cell to do its job of distributing antibodies. In order to have any effect, a Th2 cell must recognize its own antigen type on a B cell’s class II MHC. When this happens, the Th2 cell secretes cytokines which, when detected by the B cell, complete activation of the B cell, so that it may clone to memory cells and antibody-emitting effector cells. A possible reason for the complexity of this procedure will be explained shortly.
Quick (?) overview
Let’s do a run-over of the humoral immune system, the creation of antibodies[ref]Remember, we use the words immunoglobin and antibody to refer to the same things.[/ref]:
- A phagocytic cell of the innate immune system, such as a dendritic cell, phagocytozes a pathogen. It presents antigens from the pathogen on its surface in MHC II complexes.
- A B cell of the adaptive immune system whose membrane-bound immunoglobins are of a specific type happens to meet the same type of pathogen and also phagocytozes it and presents its antigens on its surface in MHC II complexes.
- Both the dendritic cell and the B cell are now APCs for the specific antigen. These two steps could have occurred in either order.
- A helper T cell for the same type of antigen bumps into our dendritic cell, binds to the MHC II complex and becomes activated.
- If the activated cell is a type Th1, it starts emitting cytokines as an alarm and to promote phagocytosis.
- If the activated cell is a type Th2, it may later meet our B cell and bind to its MHC II complex. It then release cytokines which complete activation of the B cell.
- The activated B cell duplicates itself into clones of two types, effectors and memory cells, the former being plasma cells which emit numerous immunoglobins – antibodies.
- The antibodies cling to their specific pathogens and render them harmless. At the same time, they tag them for phagocytosis.
That was the case for an external pathogen. But some pathogens, like cancers, may occur internally or, like viruses, penetrate into the cell before being noticed. Since all nucleated cells may present MHC I complexes, these will be recognized by CD8+ cells which are activated to cytotoxic T cells which will act to kill the offending (or offended) cell.
Once activated, all B and T cells duplicate to form clones of numerous effector cells and memory cells.
Why so complicated? – auto-immunity
Why do B cells have to go through a process of being activated by T cells (which have to be activated by APCs…) before producing antibodies in the primary response? One answer is to avoid auto-immunity.
Auto-immunity is when your immune system mistakes some essential part of you for a pathogen. This can lead to some really awful conditions like AIDS, lupus or myasthenia gravis. Since the receptors on B and T cells are the result of gene shuffling, many combinations will occur which could lead to auto-immunity. This is avoided in two ways:
- Under the assumption that pathogens are rare within the bone marrow and the thymus, any B or T cells which activate inside them are killed before they can get out and do any harm.
- The probability that either a B or T cell released into the body be an auto-immune cell is small (because of step 1), The process of requiring that a T cell “vet” the activation of a B cell through the APC process means that the total probability of generating an auto-immune cell is something like the product of the individual probabilities and therefore very low indeed.
So the requirement that B cells be activated by their corresponding activated T cell is a way of avoiding auto-immunity. Nevertheless, however low the probability, some auto-immune diseases do occur … alas.
Now on to embryonic development, how that sperm and egg made us.