Now we are ready to understand how it is that carbon is such a versatile element. It is at the basis of all organic chemistry and, in particular, biochemistry. The functioning of all living things depends on water and on the versatility of the carbon atom.
We saw that the carbon atom’s electron-shell configuration was
12C: 1s22s22p2
so it has four electrons in its valence shell (n=2). That enables it to share its four electrons with four others from other atoms. The bonds tend to be equally spaced around the carbon atom in the form of a tetrahedron, like those little creamer packets you get in cheap restaurants. For instance, a carbon atom can bond with four hydrogens, sharing each of its four valence electrons with one hydrogen, so each hydrogen has two and the carbon has eight and everybody is happy. This is called methane and looks like this.
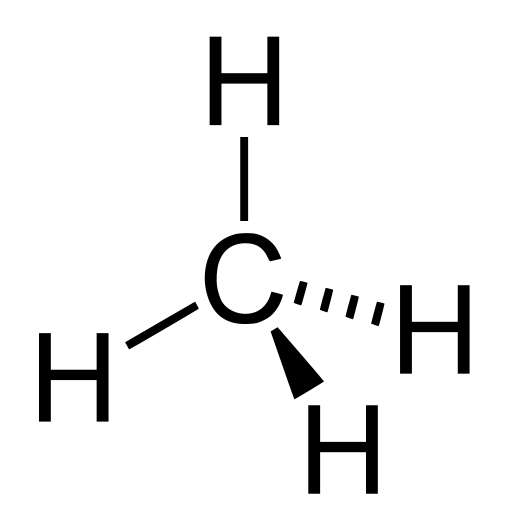
Methane molecule, CH4 by Patricia.fidi via Wikimedia Commons.
You should see one of the lower-right-hand hydrogens as pointing up out of the page; the other, down into it. The angles between any two adjacent connecting lines (which of course are only imagined by us) are about 109.5°. Carbon’s versatility in binding is illustrated by the examples in this diagram.
The dots represent valence electrons and the right-hand column is another way of looking at the product in terms of bonds rather than electrons. Each line between atoms is a shared pair of electrons. Note the double and triple inter-carbon bonds in the last two examples. This large number of ways of bonding is the key to carbon’s versatility. In fact, compared to the huge number of such molecules possible, only a relatively small number of the same biomolecules occur in living organisms. This is the first example we see of nature using the same set of techniques or tools all over the biosphere.
Single bonds between carbons also exist, of course, and have the particular advantage that the carbons and whatever is bonded to them can rotate around the axis linking the two carbons. This is more important than one might think. It turns out that some proteins function differently in their left-handed and right-handed versions. Since rotation can change the shape of the molecule, this enables biomolecules with hundreds of atoms to take on specific shapes with definite mechanical or fluid properties. (We will see some of this in the biochemistry chapter.)
The importance of water is not just because we drink it. Let’s go look at that.


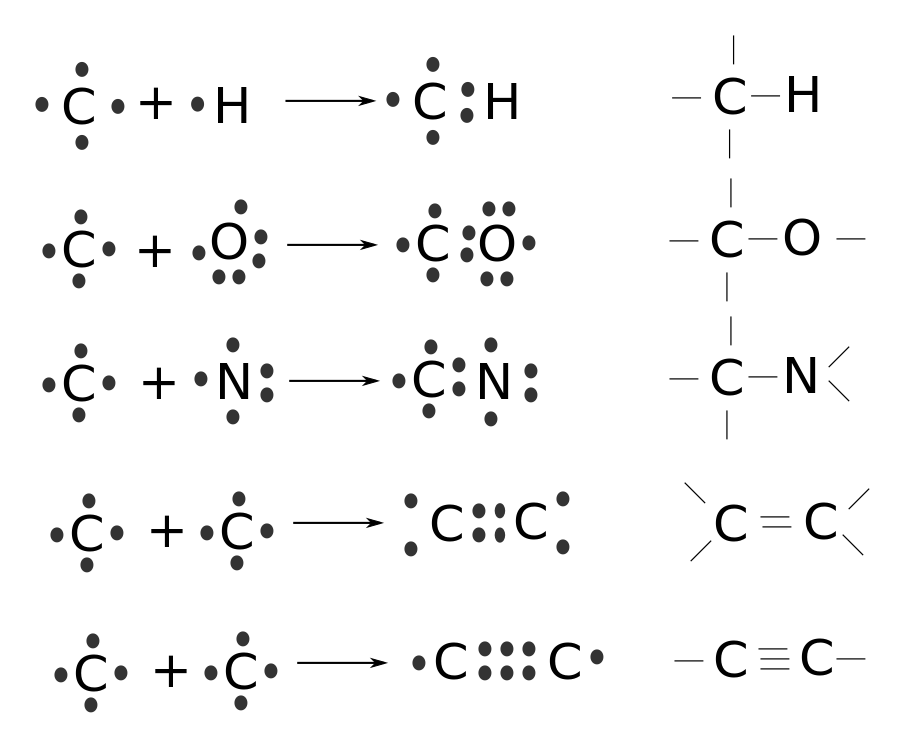

All these images are public domain, either from other such sites or by me. Those on the cheat sheet all figure elsewhere on the site and their origins are explained there. So help yourself.