Water is tremendously important to us if only because around 70% of the surface of the globe is covered with it. Each of us is 55-75% water (by weight) and life most likely arose in water. Two properties of water are of fundamental importance for biochemistry and, therefore, for life.
- the attractive force between water molecules and
- the tendency of water to ionize slightly.
Polarization and hydrogen bonds
As we saw, the electron configuration of oxygen’s eight electrons is:
16O: 1s22s22p4
So it needs two more electrons in order to fill its valence shell. As everybody knows, it bonds with two atoms of hydrogen to make H2O. Each hydrogen atom shares its electron with the oxygen, making two covalent bonds. The oxygen atom now has the desired eight electrons in its valence shell. The resulting arrangement is triangular.
Oxygen is more electronegative1Electronegativity depends on the number of electrons and on the distance of the valence electrons from the nucleus. than hydrogen, meaning it has a stronger attraction for electrons, so the electrons spend more time in the vicinity of the oxygen, making that end of the molecule slightly more negative. The molecule is said to be polarized.
Since one end of the molecule is more negative than the other, the negative end of one molecule is electrostatically attracted to the positive end of another and this forms a weak bond called a hydrogen bond. In this image, one sees the proposed tetrahedral form of the molecule as well as the hyrdrogen bonds between molecules.
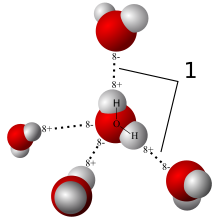
Model of hydrogen bonds between water molecules, from Wikimedia Commons
Hydrogen bonds are strongest when the electrostatic interaction of the participant atoms is maximized, as shown in the above figure. This directionality is responsible for the geometric structure of hydrogen-bonded molecules into crystals.
Hydrogen bonds do not only occur in water. They also form between an electronegative atom and a hydrogen atom covalently bonded to another electronegative atom, be it the same or different.
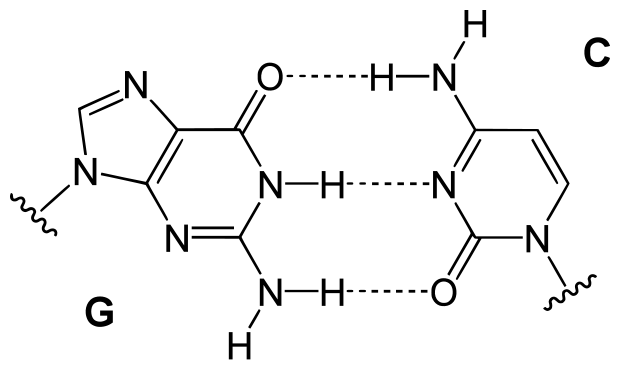
“Base pair GC” by Yikrazuul – Own work. Licensed under Public Domain via Wikimedia Commons.
Hydrogen bonds are much weaker than covalent bonds, typically on the order of a twentieth. But when there are many of them, their combined strength can be great indeed. A striking example is DNA, in which the opposing strands are held together by hydrogen bonds between the bases, as in the preceding figure. But more on that later.
If the molecules are rushing about (as in water), they are relatively independent and the substance is a liquid. Hydrogen bonds are constantly formed and broken, forming so-called “flickering clusters”. Heat them some more and they separate entirely and the water becomes a gas — water vapor. The hydrogen bonds between molecules hold them together pretty well, though, and this accounts for the rather high boiling temperature of water. Chill them down to a temperature where they do not move much any more and the hydrogen bonds assemble the molecules into a solid lattice or crystal — ice.
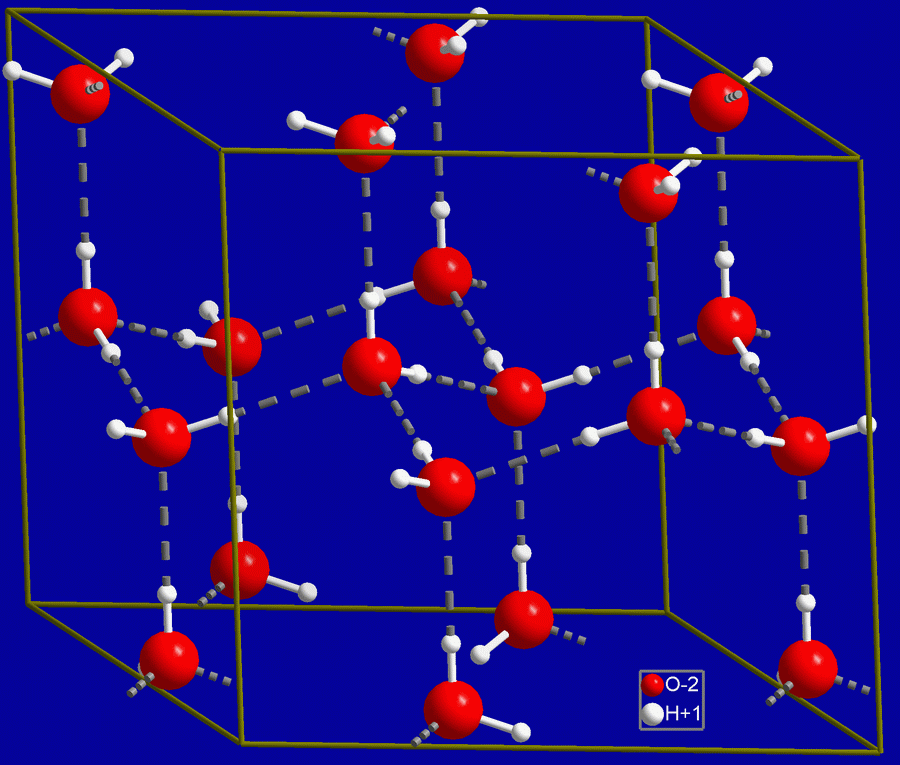
“Hex ice” by NIMSoffice (talk). via Wikipedia Commons
Ionization, hydrophobic and hydrophilic molecules
Because of its polarization, water can pull apart polar molecules, such as table salt, NaCl, where the positive sodium Na+ is attracted by the negative end of the water molecule and the negative chlorine Cl– by the positive end. This is what makes water a good solvent. One can see the advantage of this from another angle. Remember entropy? Nature wants higher entropy, meaning more disorder. But NaCl forms a highly ordered crystal structure. When the molecules are pulled apart in water, a more disordered state is achieved and entropy increases. Voilà!
On the other hand, non-polar molecules are not soluble. They are called hydrophobic, because they do not “like” water. NaCl likes it and so is called hydrophilic. This has some amazing and important consequences.
The behavior of solvents in aqueous solutions is a very important subject in biochemistry — and a fairly vast one. Let us look at one interesting and essential type of compound: Ampiphatic compounds have some regions that are polar or charged, therefore hydrophilic, and others that are not polar or charged and so are hydrophobic. In the figure below, we consider molecules illustrated as having a green hydrophilic head and long, yellow hydrophobic tails. When they are dissolved in water, the hydrophobic parts flee the water and tend to group together (like people grouped together facing outwards in the midst of a pack of threatening wolves), leaving the hydrophilic parts on the outside turned towards the water. The result is a spherical blob called a micelle.
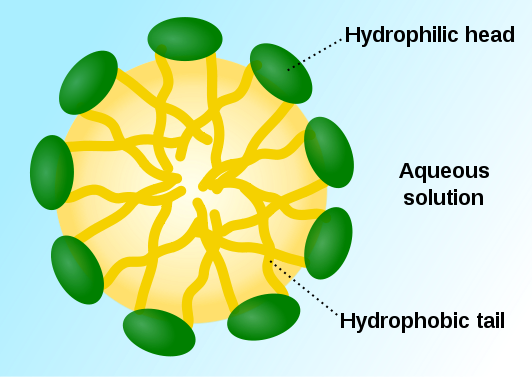
“Micelle scheme-en” by SuperManu – Own work. Licensed under CC BY-SA 3.0 via Wikimedia Commons.
One can understand this from thermodynamics, too. The water molecules are highly ordered around the hydrophobic parts of the ampiphatic molecule. Hiding these on the interior of the micelle reduces the ordering and therefore represents a state of higher entropy.2Lehninger, 49.
And there is another possibility. Think of the micelle opened up, like an orange, and flattened out and another one put alongside it, so that the hydrophobic ends are against each other and isolated from the water by the hydrophilic ends on the outside, as in part 1 of this diagram.

Lipid bilayer and micelle by Stephen Gilbert via Wikipedia Commons
This ampiphatic substance could be a lipid (organic fat), in which case this is a lipid bilayer, which is what forms cell membranes. So we are ready to start looking at cells in the physiology chapter. And all that is due to electrostatics, QM and thermodynamics — it’s all simple physics.
There are a couple more, slightly more complex, attributes of water we should know about. They are the important ideas of osmosis and buffering.



