Diffusion and osmosis
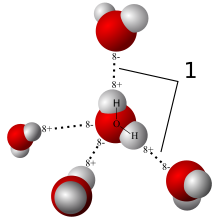
Collective or intensive properties like pressure or boiling and melting points, which are independent of the amount of a substance, are called colligative properties. The concentration of a solute is such a property. A solution wants to have the same concentration everywhere, as this represents the state of highest entropy (randomness). So in case of non-equilibrium, a solute will migrate from any region of higher concentration to regions of lower concentration — just like heat energy flows from hotter to cooler, and for similar reasons. When both regions are mixed and at the same concentration, the result is less ordered and so of higher entropy. This is diffusion.
Another very important colligative property is osmotic pressure. This is only a bit tricky to understand.
Normally, one expects a solute to diffuse from a region of higher concentration to one of lower concentration in order to bring about equal concentrations of the solute. But if the two regions are separated by a membrane which the solute cannot cross but the water can, then the opposite happens. Water flows from the region of lower concentration, i.e., where there is less solute, to the region of higher concentration, which has the effect of diluting the latter and lowering its concentration. At the same time, the solute concentration on the other (source) side goes up. This process is osmosis. The force or pressure driving the water across the membrane is called osmotic pressure.
So, in diffusion, the solute migrates; in osmosis, the solute cannot cross the membrane, so water migrates.
The concentration of solute depends not on its mass but only on the number of atoms or ions.
If the membrane is a cell membrane, then water flows into or out of the cell, depending on the solute concentration inside and outside. Cells usually have a higher solute concentration of biomolecules inside than out, which drives water into the cells. If unchecked, the inflow of water could cause the cell to expand until it exploded, but nature has come up with mechanisms to prevent this catastrophe, including reinforcement of cell walls and pumps to remove water from the cell.
In plants, osmotic pressure stiffens cells with reinforced cell walls, giving the plant rigidity to support it standing up. The opposite thing happens when a salad leaf wilts.
Buffering — acids and bases
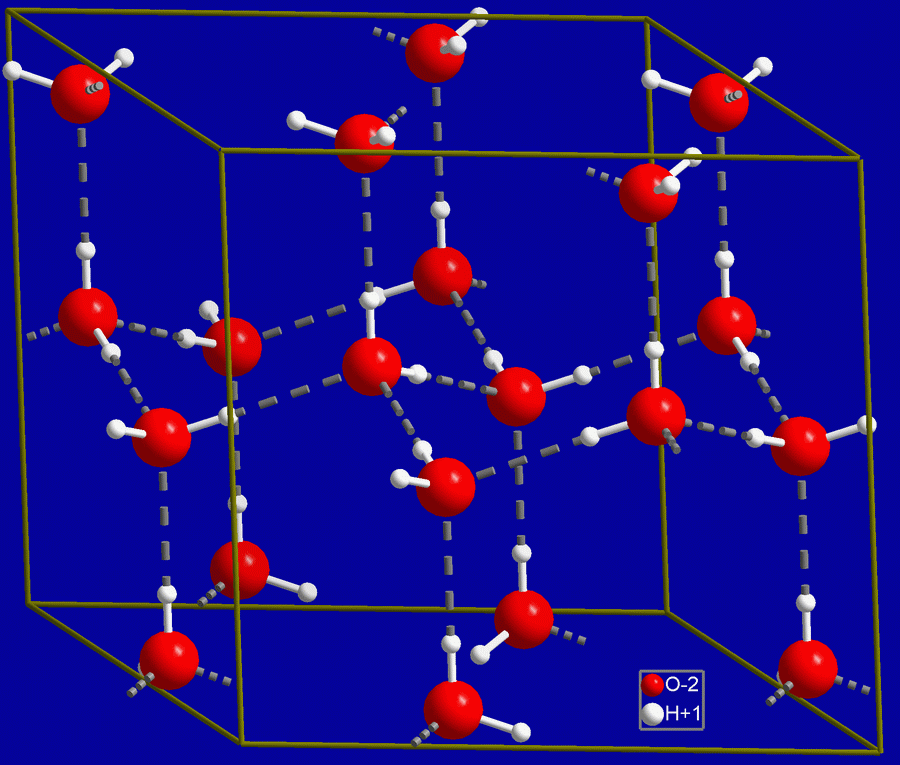
Water is naturally somewhat ionized.
There are no free protons in water (even though we often will write them as such), hence the hydronium ion, H3O+, with an extra proton. An acid is defined as a proton donor (furnishes H+) and a base as a proton acceptor (consumes H+), so it can be seen that water is weakly both: H3O+ is a donor and OH– is an acceptor. The degree of acidity is frequently indicated by the pH value, where
pH = log(1/[H+]) = -log([H+])
where [H+] is the concentration of H+ in moles per liter.1A mole is the mass of a substance contianing the same number of fundamental units (atoms, molecules, etc.) as there are atoms in 12.000 g of 12C. This number is 6.023×1023, which is called Avogadro’s number, designated by NA. Water at 25°C has a pH of 7; ph < 7 means more H+ and therefore more acidic; pH > 7 means basic. Like all such chemical transformations, there is an equilibrium point for the above reactions. This is also true for any other weak acid dissolved in water. Consider acetic acid,
CH3COOH <-> CH3OOO– + H+
which occurs in an equilibrium state of acetic acid itself (an acid, therefore a proton donor) and CH3OOO– (a base, or proton acceptor). These two substances constitute a conjugate acid-base pair. When this weak acid is dissolved in water, two equilibria must be established at the same time, for water and for acetic acid, here represented simply as HAc.
H2O <-> H+ + OH–
HAc <-> H+ + Ac–
Now if we add a small quantity of a base, say NaOH, to this solution, the base will decompose into Na+ and OH-, the latter a proton acceptor or base. This will change the pH of the solution. But the equilibrium of HAc will adjust so as to decrease the pH and the basicity. The resulting overall increase in basicity will be, in the best of cases, less than expected just considering the addition of a small quantity of strong base. Seen from the point of view of radicals, the OH- from the strong base will combine with some of the protons from the water and acetic acid. But then the acetic acid will be out of equilibrium, so it will produce more free protons to re-establish its equilibrium, thereby attenuating the effects of the added NaOH. A similar but opposite mechanism acts to maintain pH if a small quantity of strong acid is added.
This ability to reduce induced acidity is called buffering. A buffer is an aqueous system which resists changes in acidity from a small amount of added base or acid. It is composed of a weak acid and its conjugate base. It is important as the mechanism by which living beings adjust the acidity of cells. If body acidity is not within rather strict limits, enzymes will not function and so neither will we. The body uses a buffer system based on the conjugate pair carbonic acid and bicarbonate:
H2CO3 <-> H+ + HCO3–
If blood acidity starts to become too high, bicarbonate leaps in and absorbs protons. If it becomes too low, carbonic acid supplies them.2It’s really a tad more complex because of another equilibrium: H2C03 ↔ CO2 + H2O. See Lehninger, 63. This is one of many regulative mechanisms the body has for maintaining the proper equilibrium of certain solutions and processes needed by the body in order to stay alive. We will see more.
The global water cycle
Let us briefly leave the microscopic considerations of chemistry and look at water on the scale of the Earth. Water circulates through the ground, streams, oceans and lakes and the atmosphere in what is called the water cycle.

The water cycle, from USGS
This is just one of a number of transformational processes which assure the distribution of an essential component of life on Earth. The diagram is pretty much self-explanatory.
That’s it for the introductory material. Now let’s look at the history of it all. That starts in the past. Way back in the past, about 13.7 billion years ago (Gya).
Notes
| ↑1 | A mole is the mass of a substance contianing the same number of fundamental units (atoms, molecules, etc.) as there are atoms in 12.000 g of 12C. This number is 6.023×1023, which is called Avogadro’s number, designated by NA. |
|---|---|
| ↑2 | It’s really a tad more complex because of another equilibrium: H2C03 ↔ CO2 + H2O. See Lehninger, 63. |