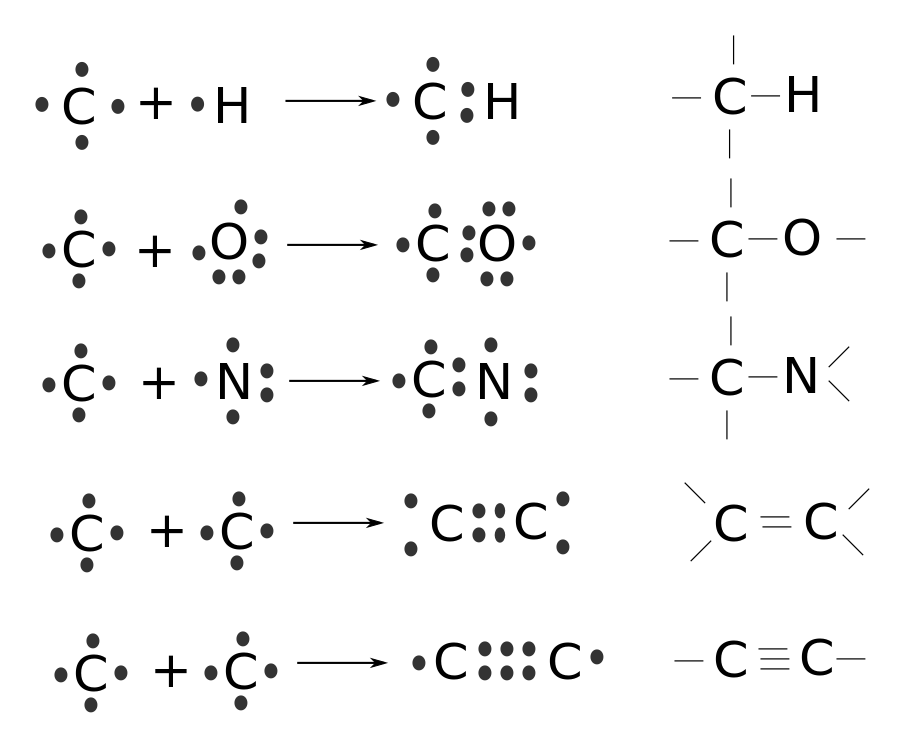Atomic structure is the basis of chemistry. It is explained by Quantum Mechanics, which is part of physics. We will see that physics explains chemistry, which explains physiology, which at least start to explain neurobiology. It’s one thing that leads to another.
In QM, the properties of a system, that is, a given object or set of objects, such as an atom, are given by the solution to the Schrödinger equation for the system. For atoms, there are a set of solutions, corresponding to different energy states of the atoms. What follows may smack of numerology.
Consider the hydrogen atom, composed of one negatively-charged electron in orbit around a nucleus containing one positively-charged proton. (This is an experimental result.) Look out, the orbit is not a well-defined path around the nucleus like those animations you see in TV ads, but rather a cloud of probability which indicates the likelihood that the electron will be found at any given point in the cloud. This is due to the probabilistic character of QM and the Uncertainty Principle. The different solutions to the Schrödinger equation express the possible energy values of the atom. Each one is specified by a set of integer numbers called quantum numbers. In the case of the hydrogen atom, they are the following:
-
The principal quantum number, designated by the symbol n, takes on integer values from 1 on up, but in practice only to 7. It indicates the shell, or level of the cloud, in which the electron is found. The values 1-7 are often indicated by the letters K, L, M…Q.
-
The orbital quantum number, l, indicates a level within the shell which is called the subshell, It can take on values from 0 up to n-1. The values 0-3 are often referred to as s, p, d and f.
-
The orbital magnetic quantum number, m, refers to the magnetic orientation of the electron. It can range from -l up through +l.
- The electron spin, ms, can take on only two values, ½ or -½.
So the only allowed values for the quantum numbers are
n = 1, 2, 3, …
l = 0…n-1 (for a given value of n)
m = -l…+l (for a given value of l)
because those are the ones for which the Schrödinger equation has solutions. It is actually quite simple.
The QM exclusion principle forbids two electrons to occupy the same state. So each set of values (n, l, m, ms) can correspond to only one electron. The result is illustrated in the following table.
|
n (shell)
|
l (subshell)
|
m (orbital)
|
Max no. electrons
|
| 1 |
0 |
0 |
2 |
| 2 |
0
1 |
0
1 |
2
6 |
| 3 |
0
1
2 |
0
-1, 0, 1
-2. -1, 0, 1, 2 |
2
6
10 |
| 4 |
0
1
2
3 |
0
-1, 0, 1
-2, -1, 0, 1, 2
-3, -2, -1, 0, 1, 2, 3 |
2
6
10
14 |
The fact that the quantum numbers do not vary continuously from, say, 0 to 0.001 and then 0.002 and on, but but jump from one integer value to another means that the energy of the electron in the electric field of the nucleus also takes on non-continuous values. These are called quantum states and are a feature, or if you prefer, a peculiarity, of QM.
The chemical properties of an atom depend only on the number of electrons. Unless the atom is chemically combined with another, the number of electrons is equal to the number of protons and is called the atomic number. All atoms except hydrogen have nuclei which also contain neutrons. As everyone knows, the number of neutrons can vary and atoms of an element with different numbers of neutrons are called isotopes. As we shall see, these are extraordinarily useful due to the fact that the properties of the element can vary from isotope to isotope.
In specifying which subshells are occupied by the electrons in an atom, one often uses the format
nl#
where l is specified as s, p, d or f and # is the number of electrons in the subshell. In its minimum energy state, called the ground state, the carbon atom (atomic number = 12, nucleus contains 6 protons and 6 neutrons) has the following electron configuration:
12C: 1s22s22p2
which indicates the maximum number of two electrons in shell 1, again in subshell s of shell 2 and the remaining two in subshell p of shell 2. Similarly, oxygen (atomic number = 16, 8 each of protons and neutrons) is
16O: 1s22s22p4
the meaning of which should now be clear.
What is interesting is that, for energetic reasons, each atom would like to have its outside subshell filled. If a few electrons are missing, it wants more; if most are missing, it might be willing to give up the rest in order to have an empty outside shell, referred to as the valence shell. (The number of electrons in this outer shell is called the valence.) For instance, hydrogen
1H: 1s1
wants two electrons or none in its 1s shell, so it could give up its electron or gain one. What happens is, two H atoms share their electrons to make a molecule of H2, so each has two electrons half the time. Better than nothing.
Since oxygen already has shell 2 half-filled, it would probably prefer to gain electrons to fill it. And carbon… but carbon is special and will be considered in a moment.
Look at sodium (Na, atomic number 11) and chlorine (Cl, atomic number 17):
Na: 1s22s22p63s1
Cl: 1s22s22p63s23p5
Sodium could happily give up that 3s electron and chlorine could use it to fill up its 3p valence shell. And this is what happens in table salt, NaCl. If you put salt in water, it separates (for reasons which will be discussed shortly) into charged ions, Na+ and Cl–, because chlorine is greedy and keeps that negative 3s electron it took away from sodium. This attraction for electrons is called electronegativity. This is very important in biochemical reactions in cells, as we shall see.
In brief, it turns out that elements with two, ten or eighteen electrons are particularly stable.
Chemistry is the study of chemical systems (atoms, molecules) and chemical bonding between such objects. In the case of NaCl, the sodium and chlorine have opposite electrical charge and the attractive electric force is what holds the molecule together. This is called ionic bonding. Sometimes, when atoms cannot decide which has more right to an electron, the electron is shared between them, as in H2, making both atoms relatively happy. Bonding based on shared electrons is called covalent bonding; it is a sort of consensus situation, if we may go on with the anthropomorphism.
Elements with the same number of electrons in their outer shells have similar chemical properties. So they are arranged in columns in that wonderful physical/chemical tool, the periodic table of the elements.
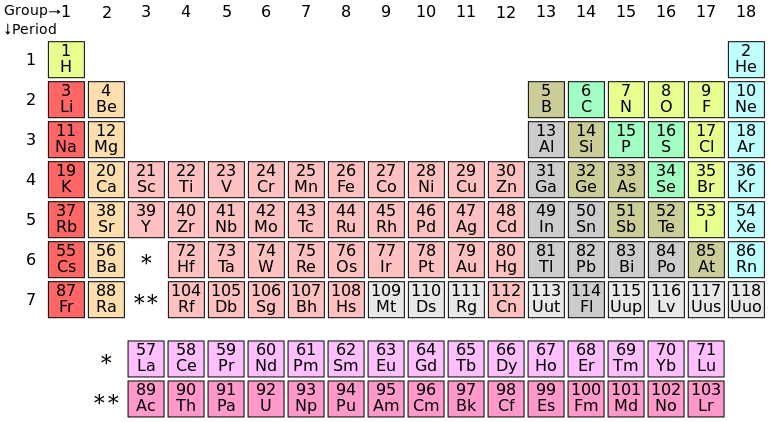
Periodic table of the elements
It is easy to see that each element in the first column is like hydrogen in having one electron in its valence shell.
H: 1s1
Li: 1s22s1
Na: 1s22s22p63s1
K: 1s22s22p63s23p64s1
… and so on.
The extra elements in the middle are rule-breakers. Instead of filling one subshell before moving on to the next, they start one, add a small number (often only one) of electrons to the next, then go back to finish filling the next-to-last.
Columns in the table are called groups; rows, periods.
The subshell configurations we have been giving are for the lowest energy state of the atom, called the ground state, in which subshells are filled from the “bottom” up (with some exceptions, as just mentioned). But if that hydrogen electron is struck by a photon, enough energy may be transferred from the photon to the 1s electron to push it into a higher-energy subshell. The atom is then said to be in an excited state. The electron may then re-descend spontaneously to the lower subshell, emitting a photon of energy equivalent to the difference in energy levels of the subshells. In QM, photons behave like waves whose energy is a function of their frequency, so the frequency – equivalently, the color – of the light emitted is characteristic of the difference in energy of the two subshells. Any atom’s subshells will therefore correspond to a given set of photon frequencies emitted and these are seen as colors, although not all these colors will be visible to a human eye. The set of frequencies constitute the spectrum of the atom and may be used to analyze the identity of a light source. In this way, we can identify the chemical components of light-emitting objects like distant stars.
There are two other types of bonding. We will consider hydrogen bonds very shortly in the discussion of water. The fourth form is due to the shifting electron density distribution around an atom. At times, this may form a temporary dipole even in a neutral atom. This may in turn induce a dipole in nearby atom in such a way that the two dipoles attract each other very weakly. This is London, or van der Waal’s, bonding.
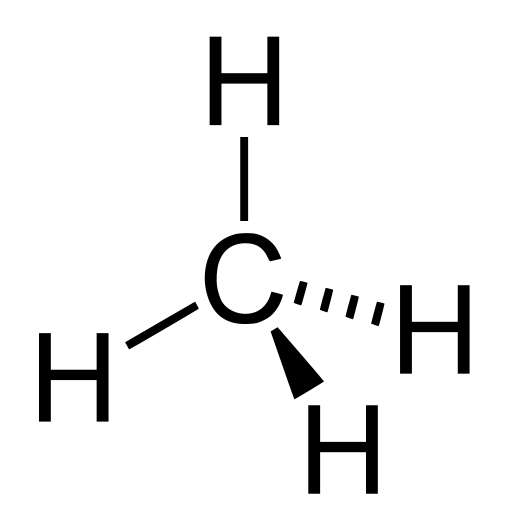
The functioning of all living things depends on water and on the versatility of the carbon atom. So let’s start with carbon.